- We tend to think of our bodies as solid, but at a microscopic level, life on Earth essentially lives in a water solution. In a manner of speaking, when life emerged from the primitive oceans on Earth, it took the water with it.
- Liquid water – H2O in liquid phase – is the critical solvent for life as known on Earth.
- Why does life require a solvent at all? This piece requires a bit of chemistry. A solvent dissolves a solute to form a solution. With ionic compounds, this means breaking down the compound into ions – such as dissolving salt (NaCl) in water, forming Na+ and Cl− ions in (liquid) solution. The ions are then free to react and form other compounds. Life requires chemical reactions, and a solvent is critical in facilitating these reactions.
- Why a liquid solvent (water or otherwise), and hence a liquid medium for life? Solids don’t work well as solvents or for facilitating chemical reactions (interaction is generally limited to surface interfaces). Also, with solids, transport of substances, which is critical to life on Earth (such as nutrients, metabolites, etc.), would be impossible. Gases, on the other extreme, are too “loose” and unconfined/unconfining/unencapsulating for such transport, for integrated cellular structures, etc. Liquids, however, generally make the best solvents, allowing chemical reactions to take place throughout the liquid. Moreover, liquids allow for effective structure and substance transport necessary for life on Earth.
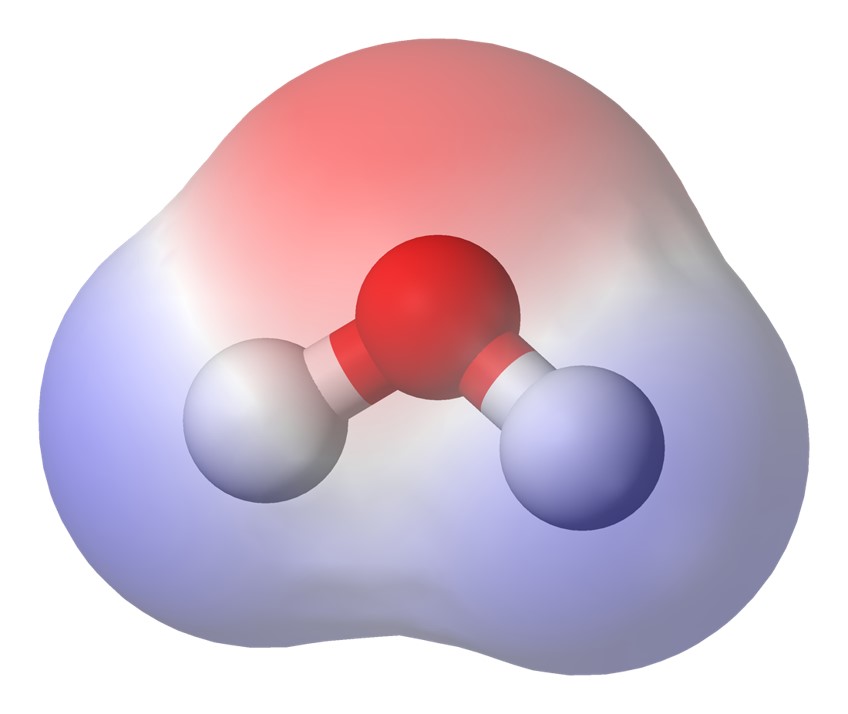
- Why liquid water? The myriad chemical reactions necessary for all Earth-based life require not just any liquid, but liquid water, with its many essential properties. Water, with its weakly polar molecular configuration, dissolves more substances than any other liquid, allowing molecular interaction and chemical reactions to take place in abundance, including reactions that are essential for life. Water is also liquid over a wide range of temperatures (at a range of pressures), which is of considerable importance for its suitability as a solvent for life (including, potentially, beyond Earth). Other advantageous properties of water include its high heat capacity (useful for temperature regulation) and its amphoteric property (allowing it to act as either an acid or a base in chemical reactions).
- In spite of liquid water’s many advantages as a solvent for life, and even though water is the only solvent for life as known on Earth, other substances could (very) hypothetically fill this role for alien life forms.
© 2015 Fosdick EDS ☾><(((°>
